Movies
A collection of movies of the wondrous world of molecular fluctuations.

A tiny water droplet
This movie shows the dynamics of a tiny droplet of 10 water molecules at room temperature, for a duration of a few picoseconds. The simulation is based on a simple model that treats water molecules as rigid. Blue dashed lines indicate hydrogen bonds between water molecules. © Yuqing Qiu
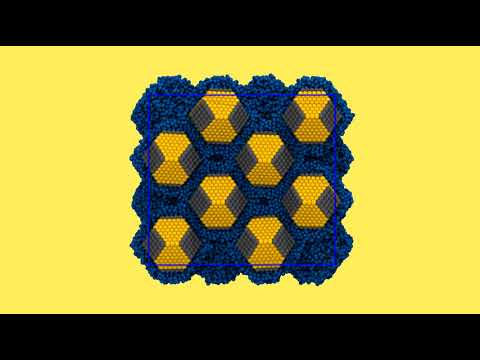
Structural transformation in a nanocrystal superlattice
This movie shows a molecular dynamics computer simulation of 32 nanocrystals (yellow and gray) covered with organic ligand molecules (blue). Nanocrystals and ligands are modeled with a simplified "coarse-grained" model that allows the computer simulation of the dynamics of many atoms. The nanocrystals form a periodic array, a so-called "superlattice". Halfway through the simulation, the superlattice changes its structure, as ligands fill the gaps between nanocrystals. Such transformations have also been observed in experiments of nanocrystal self-assembly. © Zhaochuan Fan

Birth of a covalent organic framework
This movie shows a computer simulation of the formation of a covalent organic framework (COF-5) from a solution of molecules. COFs are molecular crystals with large pores that can be used to store hydrogen gas. © Vu Nguyen
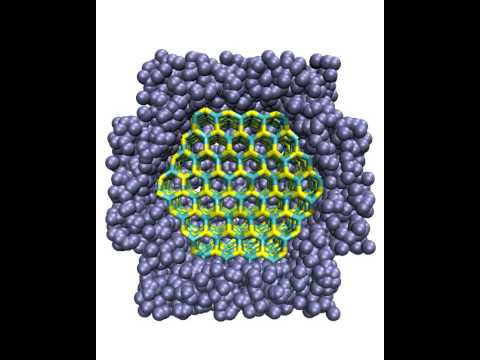
Transformation of a CdSe nanocrystal under pressure
This movie shows a molecular dynamics computer simulation of a CdSe nanocrystal (bonds between atoms are shown in yellow and blue). Initially, the nanocrystals undergoes thermal fluctuations in vacuum. Then, it is immersed in a bath of ideal gas particles (blue spheres) which is used to gradually increase the pressure to about 6 GPa. At such a high pressure, the crystal structure (called "wurtzite") becomes unstable, and the crystal transforms into the rocksalt structure.

